Decoding pre-movement neural activity from thalamic LFPs for adaptive neurostimulation in tremor patients
We studied whether brain signals can predict movement before it starts in people with tremor who have undergone deep brain stimulation surgery. Using machine learning, we showed that brain activity recorded from the thalamus and the scalp can forecast upcoming arm movements up to nearly one second in advance. Personalized models worked best, supporting tailored adaptive brain stimulation therapies.
Scientific Abstract
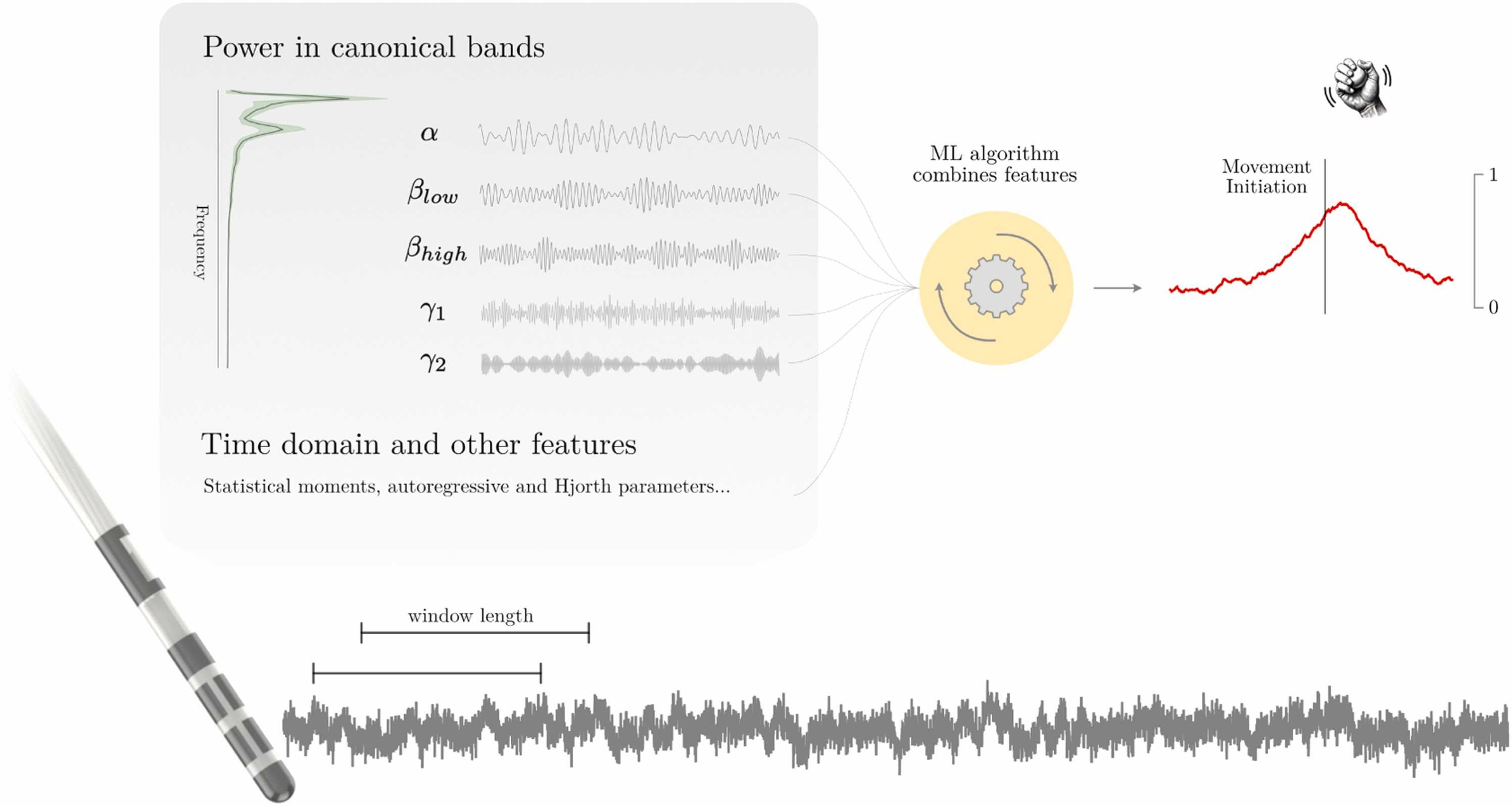
To advance adaptive deep brain stimulation for tremor disorders, we investigated the feasibility of using machine learning to decode pre-movement oscillatory changes in thalamic local field potentials (LFPs) and scalp electroencephalography (EEG) signals. Our aim was to predict upcoming upper-limb movements based on these neural signals.
ApproachWe recorded and analysed from 11 patients undergoing deep brain stimulation surgery for the treatment of tremor, employing machine learning models—including logistic regression, gradient-boosted decision trees, and convolutional neural networks—to distinguish rest periods from pre-movement periods.
Main resultsWe demonstrate that early neural correlates can predict movement onset, achieving above-chance decoding performance starting approximately 430 ms before movement initiation using thalamic LFP and 840 ms using EEG signals. Individualised, patient-specific decoders outperformed cross-patient models, reflecting inter-patient variability in neural modulatory patterns. Additionally, multiple frequency bands contributed independently to decoding performance, highlighting the importance of incorporating a spectrum of frequencies rather than relying solely on activity in any single canonical band.
SignificanceThese findings underscore the value of personalised, multi-band machine learning-based approaches for capturing the neural correlates preceding movement. They support the development of adaptive neurostimulation therapies through tailored models that account for patient-specific patterns in neural activity.
Decoding pre-movement neural activity from thalamic LFPs for adaptive neurostimulation in tremor patients
We studied whether brain signals can predict movement before it starts in people with tremor who have undergone deep brain stimulation surgery. Using machine learning, we showed that brain activity recorded from the thalamus and the scalp can forecast upcoming arm movements up to nearly one second in advance. Personalized models worked best, supporting tailored adaptive brain stimulation therapies.
Scientific Abstract
To advance adaptive deep brain stimulation for tremor disorders, we investigated the feasibility of using machine learning to decode pre-movement oscillatory changes in thalamic local field potentials (LFPs) and scalp electroencephalography (EEG) signals. Our aim was to predict upcoming upper-limb movements based on these neural signals.
ApproachWe recorded and analysed from 11 patients undergoing deep brain stimulation surgery for the treatment of tremor, employing machine learning models—including logistic regression, gradient-boosted decision trees, and convolutional neural networks—to distinguish rest periods from pre-movement periods.
Main resultsWe demonstrate that early neural correlates can predict movement onset, achieving above-chance decoding performance starting approximately 430 ms before movement initiation using thalamic LFP and 840 ms using EEG signals. Individualised, patient-specific decoders outperformed cross-patient models, reflecting inter-patient variability in neural modulatory patterns. Additionally, multiple frequency bands contributed independently to decoding performance, highlighting the importance of incorporating a spectrum of frequencies rather than relying solely on activity in any single canonical band.
SignificanceThese findings underscore the value of personalised, multi-band machine learning-based approaches for capturing the neural correlates preceding movement. They support the development of adaptive neurostimulation therapies through tailored models that account for patient-specific patterns in neural activity.
Citation
DOI
Downloads